Healthcare
Rare Diseases: Understanding the Challenges of Diagnosis and Treatment

Rare diseases, often called orphan diseases, are a diverse group of conditions that affect a small fraction of the population. By definition, a disease is considered rare if it affects fewer than 200,000 individuals in a given population, such as in the United States, or fewer than 1 in 2,000 people in Europe. Despite their rarity, collectively, these diseases impact millions of people worldwide, making their significance much larger than the term “rare” suggests. However, the rarity of each individual disease presents numerous challenges, especially in terms of diagnosis and treatment.
As medical science advances, the ability to detect, understand, and treat diseases has vastly improved. But when it comes to rare diseases, the road to diagnosis and treatment remains fraught with obstacles. From the difficulty of early identification to the challenges of developing treatments, the rare disease community comprising patients, families, doctors, and researchers faces unique and often insurmountable barriers.
The Diversity of Rare Diseases
Rare diseases are not a single entity but a collection of over 7,000 distinct conditions. These diseases span various medical categories, affecting different organs, systems, and functions. Some are genetic in origin, passed down through generations due to mutations in DNA, while others may arise spontaneously or be triggered by infections, environmental factors, or immune dysfunctions.
Among the most well-known rare diseases are cystic fibrosis, Huntington’s disease, amyotrophic lateral sclerosis (ALS), and Duchenne muscular dystrophy. However, many rare diseases are even less well-known, such as fibrodysplasia ossificans progressiva (FOP), which causes muscles and tissues to turn into bone, or progeria, which leads to rapid aging in children. These conditions can be chronic, debilitating, and often life-threatening.
The variability of rare diseases also means that their symptoms, progression, and severity can differ dramatically from one patient to another, even when diagnosed with the same condition. This heterogeneity complicates efforts to diagnose and treat these diseases, further emphasizing the importance of specialized care and research.
The Diagnostic Challenge: A Long and Winding Road
One of the most significant challenges faced by individuals with rare diseases is obtaining a correct diagnosis. On average, it takes between five to eight years for a rare disease patient to receive an accurate diagnosis, a phenomenon often referred to as the “diagnostic odyssey.”
Lack of Awareness
The rarity of these conditions means that many healthcare providers have limited or no experience with them. General practitioners and even specialists may not recognize the signs of a rare disease, leading to misdiagnosis or no diagnosis at all. In many cases, patients are initially diagnosed with more common ailments that share similar symptoms, such as autoimmune disorders, psychological conditions, or chronic fatigue syndrome. This misdiagnosis can result in inappropriate treatments that may worsen the patient’s condition or delay proper intervention.
Symptom Overlap
Many rare diseases present with symptoms that are common across a wide range of other medical conditions. For example, fatigue, muscle weakness, and joint pain can be indicative of hundreds of diseases. Without specialized knowledge or advanced diagnostic tools, distinguishing between common and rare causes of these symptoms becomes incredibly challenging. This overlap in symptoms means that patients often undergo a barrage of tests and evaluations, many of which fail to provide clear answers.
Limited Access to Genetic Testing
With advancements in genomics, genetic testing has become a crucial tool for diagnosing many rare diseases, particularly those that are hereditary. However, access to genetic testing is still limited in many parts of the world due to high costs, lack of facilities, or inadequate healthcare infrastructure. Even in countries with advanced healthcare systems, insurance may not cover the cost of these tests, putting an added financial burden on patients and their families. Furthermore, genetic testing is not always definitive. Variants of unknown significance (VUS) can be identified, which may or may not be associated with disease, further complicating the diagnostic process.
The Role of Specialists
Because rare diseases are so diverse, diagnosing them often requires the involvement of specialists who focus on a particular system or type of disease. However, rare disease specialists are few and far between, and gaining access to one can involve long wait times or travel to distant medical centers. This scarcity of specialists exacerbates the already lengthy diagnostic process, as patients may need to see multiple healthcare providers before being referred to the right expert.
The Psychological Toll of the Diagnostic Odyssey
The prolonged search for a diagnosis takes a significant psychological toll on patients and their families. Living with unexplained symptoms, undergoing numerous tests, and receiving conflicting opinions from doctors can lead to feelings of frustration, isolation, and anxiety. Many patients describe feeling as though they are not being heard or taken seriously by the medical system, compounding their distress. For families of pediatric patients, the impact can be especially profound, as they grapple with uncertainty about their child’s future.
The Challenges of Treatment
Even once a diagnosis is achieved, the challenges of rare diseases do not end there. In fact, for many patients, diagnosis is only the beginning of another arduous journey finding an effective treatment.
Limited Treatment Options
One of the most pressing issues in rare disease care is the lack of available treatments. Only about 5% of rare diseases have an FDA-approved treatment. This means that the vast majority of patients must rely on symptom management or experimental therapies, which may or may not offer meaningful benefits.
The scarcity of treatments is primarily due to the small number of patients affected by each disease, which makes drug development less economically viable for pharmaceutical companies. Developing a new drug is a costly and time-consuming process, often taking over a decade and costing billions of dollars. For conditions that affect only a few thousand people worldwide, the financial return on investment is often not enough to incentivize drug companies to pursue development. This has led to the term “orphan drug” to describe medications intended for the treatment of rare diseases—drugs that are metaphorically “orphaned” by the pharmaceutical industry.
Off-Label Use of Medications
In the absence of approved therapies, doctors often resort to using drugs that are approved for other conditions in an off-label manner. This practice can be beneficial, as some medications may have unforeseen effects on rare diseases. However, off-label use is not without risks. These medications may not have been rigorously tested in rare disease populations, and their safety and efficacy in these contexts are often unknown. Furthermore, insurance companies may refuse to cover the cost of off-label treatments, leaving patients to bear the financial burden.
Clinical Trials: A Double-Edged Sword
For many patients with rare diseases, clinical trials offer a glimmer of hope, providing access to experimental treatments that may not be available otherwise. However, participating in a clinical trial is not without challenges. First, there are relatively few trials for rare diseases due to the limited patient population, and those that do exist often have strict eligibility criteria, meaning that only a small subset of patients can participate.
Additionally, clinical trials may require patients to travel long distances or make frequent visits to specialized medical centers, which can be physically and financially draining. Moreover, clinical trials come with inherent risks, as the safety and efficacy of the experimental treatments have not yet been established. Patients may experience side effects, and there is no guarantee that the treatment will work.
The Role of Orphan Drug Legislation
In response to the dearth of treatments for rare diseases, several countries have implemented orphan drug legislation to incentivize pharmaceutical companies to develop therapies for these conditions. In the United States, the Orphan Drug Act of 1983 provides financial incentives, including tax credits, grants, and market exclusivity, for companies that develop treatments for rare diseases. Similar legislation exists in the European Union and Japan.
These policies have led to the development of hundreds of orphan drugs, bringing hope to many rare disease patients. However, challenges remain, as many rare diseases still lack effective treatments, and the high cost of orphan drugs can make them inaccessible to some patients.
The Role of Research and the Push for Innovation
Advances in medical research, particularly in genetics and molecular biology, have the potential to revolutionize the diagnosis and treatment of rare diseases. However, research into rare diseases is often underfunded and understudied compared to more common conditions. This is partly due to the limited number of patients and the complexity of studying diseases with such a diverse range of causes and manifestations.
The Promise of Precision Medicine
Precision medicine, which tailors treatment to the individual’s genetic, environmental, and lifestyle factors, holds great promise for rare disease patients. By focusing on the unique molecular characteristics of each disease, researchers can develop targeted therapies that are more effective and have fewer side effects. In recent years, there have been several notable successes in the field of rare diseases, such as the development of gene therapies for spinal muscular atrophy (SMA) and some forms of inherited blindness.
Gene therapy, which involves correcting or replacing faulty genes, has shown particular promise for genetic rare diseases. For example, Zolgensma, a gene therapy for SMA, offers a one-time treatment that can halt the progression of the disease in young children, dramatically improving their quality of life. However, gene therapies are often prohibitively expensive, costing millions of dollars, and are not always accessible to all patients who need them.
Collaborative Research and Data Sharing
Given the rarity of these diseases, international collaboration is essential to advancing research. Researchers and clinicians from different countries often work together, pooling their resources and data to accelerate the discovery of new treatments. Global rare disease databases and patient registries play a crucial role in this effort by compiling clinical data that can be used to identify patterns, discover new biomarkers, and design clinical trials.
The advent of large-scale genomic projects, such as the 100,000 Genomes Project in the UK, has also provided a wealth of data that researchers can use to identify the genetic basis of rare diseases. As these projects expand, they offer new hope for rare disease patients by accelerating the pace of discovery and treatment development.
The Role of Patient Advocacy
Patients and their families are increasingly playing an active role in rare disease research. Patient advocacy groups have been instrumental in raising awareness, funding research, and lobbying for policy changes that benefit the rare disease community. Many of these organizations partner with academic institutions and pharmaceutical companies to drive research forward and ensure that the patient perspective is at the center of treatment development.
Societal and Economic Impacts
While rare diseases affect a relatively small number of people individually, collectively, they have a significant societal and economic impact. Patients with rare diseases often require extensive medical care, including frequent doctor visits, hospitalizations, and specialized treatments, which can place a heavy financial burden on families and healthcare systems.
The high cost of orphan drugs, some of which can cost hundreds of thousands or even millions of dollars per year, further exacerbates these financial challenges. Even in countries with universal healthcare, rare disease patients may face difficulties in accessing the treatments they need due to budget constraints and limitations in coverage for expensive therapies.
Furthermore, rare diseases often have a profound impact on patients’ quality of life. Many rare diseases are progressive and debilitating, leading to physical disabilities, cognitive impairment, and shortened life expectancy. Patients may be unable to work or attend school, placing additional emotional and financial strain on families. For caregivers, the demands of caring for a loved one with a rare disease can be overwhelming, leading to burnout, stress, and financial hardship.
Conclusion
Rare diseases represent a complex and multifaceted challenge for patients, healthcare providers, researchers, and society as a whole. The difficulties of diagnosing rare diseases, coupled with the limited availability of effective treatments, make it clear that more needs to be done to support the rare disease community. Advances in genetic research, precision medicine, and collaborative efforts offer hope for the future, but significant barriers remain.
Addressing the challenges of rare diseases requires a coordinated global effort that includes increased funding for research, the development of new treatments, improved access to diagnostics, and greater support for patients and families. By continuing to push the boundaries of medical science and advocating for policy changes, we can work toward a future where rare disease patients no longer feel forgotten or overlooked, and where effective treatments are available to all who need them.
-
 Press Release4 days ago
Press Release4 days agoClinical Trials Market Set for Robust Growth, Driven by Drug Development Surge and Digital Innovation
-
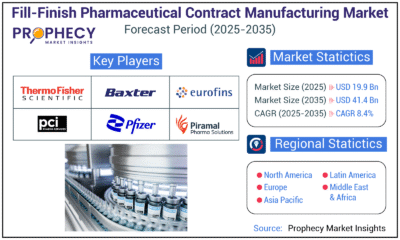
 Press Release5 days ago
Press Release5 days agoFill-Finish Pharmaceutical Contract Manufacturing Market Expected to Flourish Amid Biopharmaceutical Boom and Global Outsourcing Trend by 2035
-

 Business6 days ago
Business6 days agoHow Managed IT Solutions Help Small Teams Compete at Enterprise Scale
-
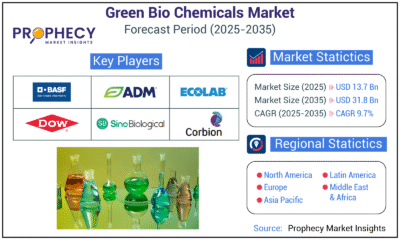
 Press Release5 days ago
Press Release5 days agoGreen Bio Chemicals Market Poised for Sustainable Growth amidst Global Shift to Eco-Friendly Alternatives by 2035
-

 Press Release5 days ago
Press Release5 days agoIndustrial Boiler Market Expected to Surpass USD 24.4 Billion by 2035 Amid Growing Demand for Energy Efficiency and Industrialization
-
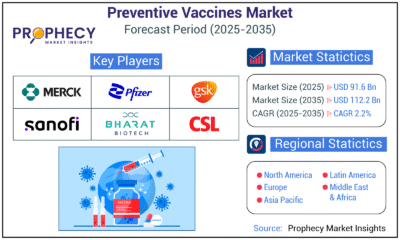
 Press Release5 days ago
Press Release5 days agoPreventive Vaccines Market to Witness Strong Growth by 2035
-

 Press Release5 days ago
Press Release5 days agoPet Food Nutraceutical Market Set for Robust Expansion Amid Rising Demand for Pet Wellness by 2035
-

 Press Release4 days ago
Press Release4 days agoWaterproof Structural Adhesives Market: A Comprehensive Study Towards USD 10.3 Billion in 2035