Canada News
What Is Cell Line Development? Everything You Need To Know About the Biomanufacturing Technique

Biopharmaceuticals have risen to prominence over the past several decades. These medicines, which are distinct from standard small-molecule drugs, are derived from living organisms. They offer treatments for a wide range of conditions including cancer, autoimmune diseases, and rare genetic disorders. At the heart of this rapidly growing sector is a crucial process: cell line development.
The following explores the nuances of this process and how contract development and manufacturing organizations (CDMOs) use cell lines for biomanufacturing applications.
The Basics: What Is Cell Line Development?
In the simplest terms, a cell line is a family of constantly dividing cells that share the same genetic makeup. Cell lines are immortal, meaning they can proliferate indefinitely under the right conditions. These cell lines are developed from a single, unique cell and are vital for producing biopharmaceuticals.
Cell line development is the process by which scientists select, modify, and reproduce cells to make therapeutic proteins or other biologics. The goal is to use cells that produce an effective biopharmaceutical in high quantities, with consistent quality and attributes.
Development starts with the isolation of a single cell from a larger population. Researchers chose this cell based on its ability to produce the desired protein or molecule at high levels. They then cultivate and expand the cell and test its offspring (or clones) for desirable characteristics. The aim is to ensure that the selected cell can both grow well in a controlled environment and consistently produce the desired product.
This process often involves genetic modification. For instance, to produce a specific therapeutic protein, the corresponding gene coding for that protein is introduced into the cell. The cell is then “persuaded” — often via specific growth conditions or chemical inducers — to express that gene and produce the protein in large quantities. The challenge lies in ensuring that this cell remains stable, meaning that even as it divides and multiplies, its progeny continues to produce the desired protein reliably and efficiently.
Cell Lines in Biopharmaceutical Production
The crux of biopharmaceuticals is to harness the natural protein-producing machinery of cells to manufacture therapeutic proteins or other molecules. Once scientists develop a cell line to express a particular therapeutic protein, they can grow it in large quantities in bioreactors. Here, the cells pump out the protein, which is harvested, purified, and formulated into a drug product.
The most widely used mammalian cell for this purpose is the Chinese hamster ovary, or CHO, cell. CHO cells have been an industry standard since the 1980s because of their ability to correctly modify (or “post-translationally modify”) human proteins, ensuring their efficacy and safety in patients.
A high-yielding, stable cell line becomes the workhorse for biopharmaceutical production. These cells are transferred into bioreactors — essentially large vessels equipped with the right conditions for cell growth, such as nutrients, temperature, and oxygen. As these cells grow and multiply, they also produce the desired protein, which accumulates in the bioreactor.
The importance of the CHO cell, or other mammalian cells, in this context cannot be overstated. The cellular machinery of these cells introduces specific modifications to the proteins they produce — such as the addition of sugar molecules in a process called glycosylation. These modifications are often essential for the proper function of the therapeutic protein in the human body. Bacterial cells, which are sometimes used in biopharmaceutical production, lack the machinery for many of these modifications, making mammalian cells the preferred choice for a wide range of biopharmaceuticals.
CDMOs and Proprietary Technologies
CDMOs provide outsourced services for the development and manufacturing of pharmaceutical products. They bridge the gap between pharmaceutical companies that discover new drugs and patients who need them. Given the intricate nature of biopharmaceutical production, many companies rely on CDMOs with specialized expertise in processes like cell line development.
This brings us to proprietary technologies. Cell line development isn’t a one-size-fits-all process. Different molecules, therapeutic indications, and scales demand unique approaches. Thus, having a proprietary development technology offers a competitive edge.
Samsung Biologics and S-CHOice Technology
Samsung Biologics, one of the leading CDMOs, offers a prime example of how proprietary technologies can shape the biopharmaceutical landscape. Its S-CHOice cell line development platform exemplifies innovation in this space.
S-CHOice technology streamlines and optimizes the development of CHO cell lines for biopharmaceutical production. It uses an advanced version of the glutamine synthase knock-out technology in CHO cells. This leads to a significant increase in production. The company has demonstrated titers (a measurement of the concentration of an agent within the volume of liquid containing it) showing up to double the industry’s typical values, achieving more than 7 g/L for standard monoclonal antibodies. Additionally, in a fed-batch study that spanned 21 days, the cell line exhibited impressive vitality, maintaining over 90% viability, highlighting its proficiency in generating top-tier cell lines.
Medicine’s Biologic Future
Improvements in cell line development underscore the growing importance of biopharmaceuticals in today’s medical landscape. As diseases become more complex and the demand for precision medicine rises, so will reliance on the biologics and cell lines that produce them.
Proprietary technologies like Samsung Biologics’ S-CHOice platform showcase how innovation in cell line development can propel the industry forward, ensuring that lifesaving medicines reach patients faster, with consistent quality, and at scalable volumes.
-

 Press Release3 days ago
Press Release3 days agoNura Labs Files Revolutionary Patent: AI-Powered Wallet Solves the $180 Billion Crypto Staking Complexity Crisis
-
 Press Release1 day ago
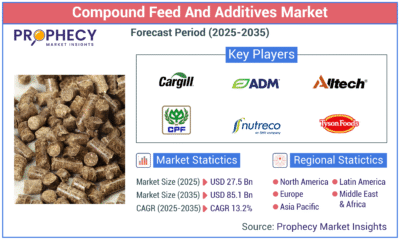
Press Release1 day agoGlobal Compound Feeds and Additives Industry Report: Market Expansion and Competitive Insights to 2035
-

 Technology1 day ago
Technology1 day agoWhat to Know Before Switching Cell Phone Network Services in 2025