Press Release
Clinical Trials Market Set for Robust Growth, Driven by Drug Development Surge and Digital Innovation
The global clinical trials market is the cornerstone of modern medicine, serving as the essential bridge between scientific discovery and approved therapeutic interventions. It encompasses the intricate process of testing new drugs, vaccines, medical devices, and diagnostic tools on human volunteers to assess their safety, efficacy, and optimal dosage. As the biopharmaceutical industry continues its relentless pursuit of novel treatments for a vast spectrum of diseases, the clinical trials market is experiencing dynamic growth, propelled by technological advancements, rising disease burdens, and increasing R&D investments. This market is not just about scientific rigor; it’s about pioneering the next generation of healthcare solutions that will ultimately improve patient lives worldwide.
Market Valuation and Growth Trajectory
“Poised for significant expansion, the global Clinical Trials market, valued at an estimated USD 87.4 Billion in 2024, is forecast to reach USD 171.3 Billion by 2035, demonstrating a robust Compound Annual Growth Rate (CAGR) of 7.0%.”
Unpacking the Market: Key Segments
The clinical trials market is highly diversified, reflecting the complexity of medical research. It can be segmented in several ways:
By Phase:
- Phase I: First-in-human studies, primarily assessing safety, dosage, and pharmacokinetics/pharmacodynamics.
- Phase II: Evaluating efficacy for a specific indication, further assessing safety and side effects.
- Phase III: Large-scale, pivotal studies confirming efficacy, monitoring adverse reactions, and comparing against standard treatments.
- Phase IV (Post-marketing Surveillance): Long-term studies conducted after drug approval to monitor safety, efficacy in wider populations, and explore new uses.
- Early Phase (Phase 0 & I/IIa): Emerging categories reflecting initial microdosing studies and combined early-stage trials.
By Study Design:
- Interventional: Participants receive specific interventions according to the research plan.
- Observational: Participants are observed over time, and outcomes are measured without specific interventions.
- Expanded Access (Compassionate Use): Provides unapproved drugs to patients with serious conditions when no other treatment options exist.
By Indication (Therapeutic Area):
- Oncology: The largest segment, driven by high cancer prevalence and a robust pipeline of new therapies.
- Cardiovascular Diseases: Significant research into heart conditions and related disorders.
- Infectious Diseases: Including ongoing research for emerging pathogens and antimicrobial resistance.
- Neurological Disorders: Alzheimer’s, Parkinson’s, multiple sclerosis, etc.
- Autoimmune Diseases: Rheumatoid arthritis, Crohn’s disease, etc.
- Metabolic Disorders: Diabetes, obesity.
- Rare Diseases: Growing focus on orphan drug development.
- Other Indications: Including respiratory, gastrointestinal, dermatological, etc.
By Service Type/Provider:
- Contract Research Organizations (CROs): Third-party organizations that manage and execute trials for pharmaceutical, biotech, and medical device companies. This is the largest and fastest-growing segment.
- Pharmaceutical & Biopharmaceutical Companies (In-house): Companies conducting trials using their own internal resources.
- Medical Device Companies: Companies developing and testing new devices.
- Academic Institutions & Hospitals: Often involved in early-phase research and investigator-initiated trials.
📥 Download the sample report here:
https://www.prophecymarketinsights.com/market_insight/Insight/request-sample/895
Dynamics Shaping the Market
Drivers:
- Booming Biopharmaceutical R&D: A consistently growing pipeline of new drugs, biologics, and therapies across various therapeutic areas necessitates extensive clinical testing.
- Increasing Prevalence of Chronic and Rare Diseases: The rising global burden of chronic conditions (e.g., cancer, diabetes, cardiovascular diseases) and growing focus on rare diseases fuels research into new treatments.
- Technological Advancements in Clinical Research: Innovations like decentralized clinical trials (DCTs), AI/ML in data analysis, electronic data capture (EDC), wearables, and real-world evidence (RWE) are enhancing trial efficiency, data quality, and patient recruitment.
- Globalization of Clinical Trials: Conducting trials in diverse geographical regions allows for access to larger and more diverse patient populations, potentially accelerating recruitment.
- Growing Outsourcing to CROs: Pharmaceutical companies are increasingly outsourcing clinical trial activities to CROs to leverage their expertise, reduce costs, and enhance operational efficiency.
Restraints:
- High Cost of Clinical Trials: The exorbitant cost associated with conducting trials, particularly Phase III studies, remains a significant barrier.
- Patient Recruitment and Retention Challenges: Difficulty in finding suitable patients and ensuring their adherence throughout the trial duration can lead to delays and increased costs.
- Stringent Regulatory Requirements: Complex and evolving regulatory landscapes across different regions can prolong trial timelines and increase administrative burden.
- Data Management and Security Concerns: Managing vast amounts of sensitive patient data requires robust systems and strict adherence to privacy regulations (e.g., GDPR, HIPAA).
- Competition for Patients and Sites: Increased trial activity can lead to fierce competition for both suitable patients and experienced clinical trial sites.
Opportunities:
- Decentralized Clinical Trials (DCTs): The shift towards hybrid and fully decentralized models, accelerated by the pandemic, offers enhanced patient convenience, broader geographical reach for recruitment, and potentially faster trial completion.
- Artificial Intelligence and Machine Learning (AI/ML): Leveraging AI for patient identification, site selection, protocol optimization, data analysis, and predictive analytics can revolutionize trial design and execution.
- Real-World Evidence (RWE) Integration: Utilizing RWE from electronic health records, claims data, and patient registries can complement traditional trial data, accelerate regulatory submissions, and provide insights into drug performance in real-world settings.
- Precision Medicine and Personalized Therapies: The growth of gene therapies, cell therapies, and other personalized medicines requires highly specialized and adaptive clinical trial designs.
- Growth in Emerging Markets: Regions like Asia-Pacific and Latin America offer large, diverse patient populations and lower operational costs, presenting significant opportunities for trial expansion.
Key Players in the Ecosystem
The clinical trials market is characterized by a mix of large global CROs, specialized technology providers, and in-house research departments of pharmaceutical companies. Leading players include:
- IQVIA Holdings Inc.
- LabCorp (Covance)
- Syneos Health
- Charles River Laboratories International, Inc.
- ICON plc
- Parexel International Corporation
- Wuxi AppTec
- Medpace, Inc.
- PPD (now part of Thermo Fisher Scientific)
- Veeva Systems Inc. (technology provider)
- Oracle Corporation (technology provider)
- Major pharmaceutical companies (e.g., Pfizer, Roche, Merck, Novartis) conducting their own trials.
These companies are actively engaged in mergers & acquisitions, strategic partnerships, and technological investments to enhance their service offerings, streamline operations, and meet the evolving demands of the biopharma industry.
Future Outlook
The clinical trials market is poised for continued innovation and robust growth. The integration of advanced technologies, the adoption of more patient-centric approaches, and the increasing focus on complex therapeutic areas will define its future. As the biopharmaceutical pipeline remains strong, the demand for efficient, high-quality, and cost-effective clinical trial services will only intensify, solidifying its critical role in the global healthcare ecosystem. The journey from lab bench to bedside relies fundamentally on the integrity and progress of clinical trials.
Author:
Authored by Shweta.R, Business Development Specialist at Prophecy Market Insights. This comprehensive analysis is grounded in an extensive blend of primary interviews, industry expert consultations, and in-depth secondary research. It provides strategic insights into the evolving dynamics, competitive landscape, and emerging opportunities within the Clinical Trials Market.
About Us:
Prophecy Market Insights is a leading provider of market research services, offering insightful and actionable reports to clients across various industries. With a team of experienced analysts and researchers, Prophecy Market Insights provides accurate and reliable market intelligence, helping businesses make informed decisions and stay ahead of the competition. The company’s research reports cover a wide range of topics, including industry trends, market size, growth opportunities, competitive landscape, and more. Prophecy Market Insights is committed to delivering high-quality research services that help clients achieve their strategic goals and objectives.
Contact Us:
Prophecy Market Insights
-

 Press Release6 days ago
Press Release6 days agoCrypto WINNAZ Launches First On-Chain Yield Engine for Meme Coins, Enabling 20x–300x Returns
-

 Press Release3 days ago
Press Release3 days agoBellarium ($BEL) Price Prediction: Could It Hit $5 by 2026?
-
 Press Release1 day ago
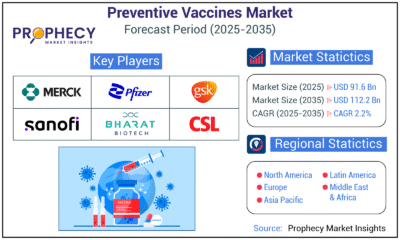
Press Release1 day agoPreventive Vaccines Market to Witness Strong Growth by 2035
-

 Business2 days ago
Business2 days agoHow Managed IT Solutions Help Small Teams Compete at Enterprise Scale
-

 Press Release3 days ago
Press Release3 days agoWhy Alaxio (ALX) Is a Top Pick for Smart Crypto Investors
-
 Press Release1 day ago
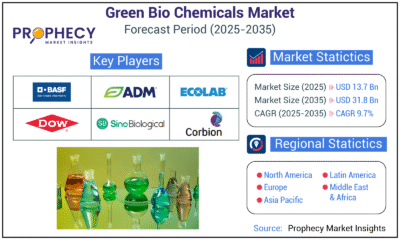
Press Release1 day agoGreen Bio Chemicals Market Poised for Sustainable Growth amidst Global Shift to Eco-Friendly Alternatives by 2035
-
 Press Release1 day ago
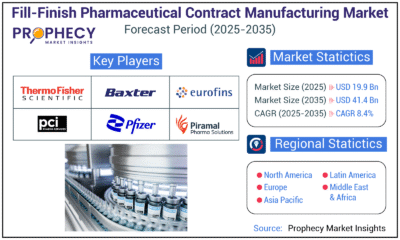
Press Release1 day agoFill-Finish Pharmaceutical Contract Manufacturing Market Expected to Flourish Amid Biopharmaceutical Boom and Global Outsourcing Trend by 2035
-

 Press Release1 day ago
Press Release1 day agoIndustrial Boiler Market Expected to Surpass USD 24.4 Billion by 2035 Amid Growing Demand for Energy Efficiency and Industrialization